GS1 Industry Standards
GS1 standards are open, global, proven and simple.
We have placed cookies on your browser to help make this website better. By continuing to browse or by clicking “Accept All Cookies,” you agree to the storing of first- and third-party cookies on your device to enhance your experience, analyze site usage, and assist in our marketing efforts. You can change your cookie settings at any time. Cookie Policy >
GS1 standards are open, global, proven and simple.
MicroPort complies with UDI requirements through the GS1 system of standards which provides a more efficient and responsive way of trading to our customers. Distinct numbers are used in this system to identify goods, services, assets and locations worldwide. These numbers can be represented in barcodes to enable their electronic reading wherever required in business processes.
GS1 Identification Keys include:
Adoption of GS1 in the health care industry is intended to:
For more information about GS1 standards, access the GS1 global site: https://www.gs1.org/.
When GS1 labels are not present, UDI information can be derived by combining part numbers or descriptions of devices with a cross referencing tool.
Listed below are multiple ways to obtain a device’s GTIN from its catalog numbers or descriptions when labels are not present.
MicroPort began placing GTIN barcodes on labeling in June 2019. Please note that previously existing inventory may have labels that are not yet compliant.
The images below compare MicroPort labels before and after the addition of GS1 barcodes.
| MicroPort Orthopedics' Pre-GS1 Label | MicroPort Orthopedics' Post-GS1 Label |
|---|---|
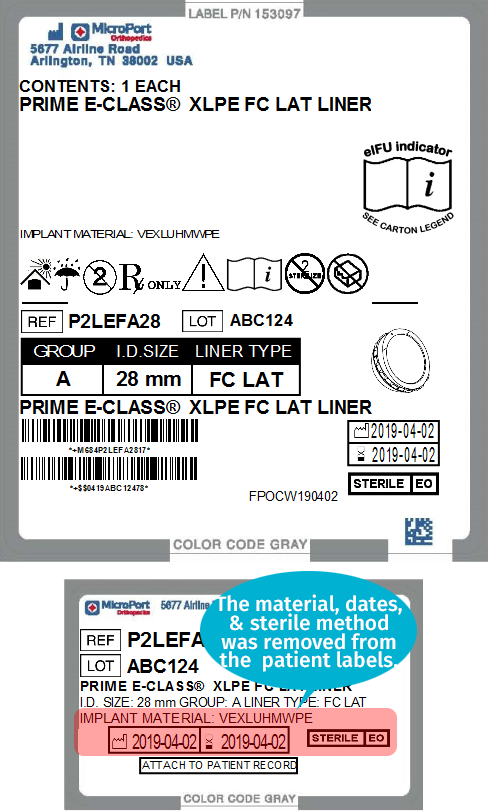 |
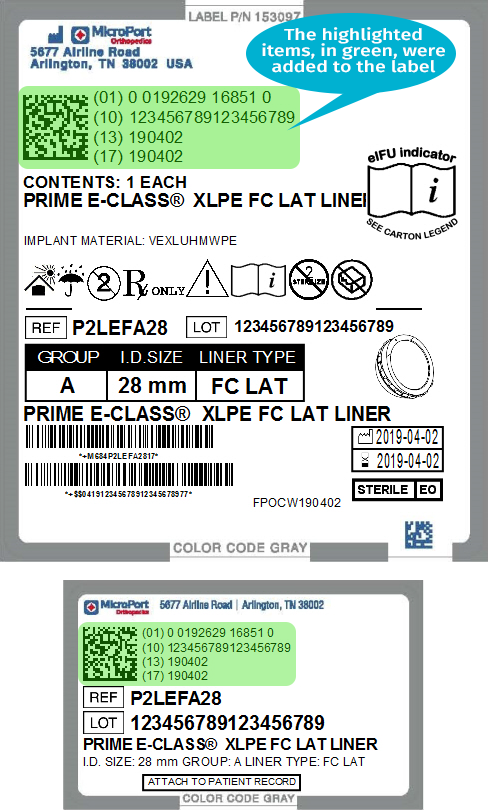 |
Direct Part Marking requires the UDI (GTIN) be permanently added to reusable and reprocessed devices at the point of manufacture.
More specifically, a medical device must have a Direct Part Marking if the three requirements below are met:
This will enable traceability of these devices after they are separated from their labeling or packaging.
Class I reusable devices make up the majority of our products that need to be marked.
The FDA deadline for new inventory Class I reusable devices is September of 2020. The FDA deadline for existing inventory for Class I reusable devices is September of 2023.
MicroPort Orthopedic’s reusable instruments are directly marked with the part number and lot number.
These two values can be used to construct the UDI DI (device identifier) and UDI PI (production identifier).
The DI can be constructed using the part number and the PI uses the lot number.
No additional PI elements are associated to the reusable instruments, other than the lot number.
Identify part number on the instrument.
Example: E5002001
Construct UDI DI based on the following format M684 + [PART NUMER] + 1.
Example: UDI DI M684E50020011
Identify lot number.
Example: ABC124
The UDI PI will be the lot number. No additional PIs are associated to reusable instruments for MPO.
Example: UDI PI ABC124
Surgeons in over 70 countries utilize MicroPort Orthopedics Hip & Knee products. Our national sales team is ready to answer any question you have.
